Clinical trials are the cornerstone of medical advancements, rigorously testing new treatments and technologies before they reach patients. These trials, meticulously designed and ethically conducted, follow a structured process involving various phases, each with specific objectives and participant groups. Understanding the intricacies of clinical trials is crucial for appreciating the journey from laboratory discovery to life-saving therapies.
From Phase I, focusing on safety and dosage in a small group of volunteers, to Phase III, evaluating efficacy and comparing the new treatment against existing options in a larger, more diverse population, each stage plays a vital role. The rigorous data analysis and ethical oversight ensure the safety and well-being of participants while generating evidence-based results that inform healthcare decisions globally.
The impact extends beyond individual patients, shaping healthcare systems and influencing future medical research.
Introduction to Clinical Trials
Clinical trials are the cornerstone of medical advancements, providing rigorous scientific evidence to evaluate the safety and effectiveness of new medical interventions, including drugs, devices, and therapies. They are essential for ensuring that treatments are both beneficial and safe for patients, ultimately improving healthcare outcomes. The process involves carefully designed studies conducted in a controlled environment, adhering to strict ethical guidelines and regulatory requirements.
The importance of clinical trials cannot be overstated. They provide the data necessary for regulatory agencies like the FDA (in the US) and EMA (in Europe) to approve new treatments for widespread use. Without robust clinical trial data, new medications and therapies would not be available to the public. Furthermore, clinical trials often contribute to a deeper understanding of diseases, leading to the development of improved diagnostic tools and more effective treatment strategies.
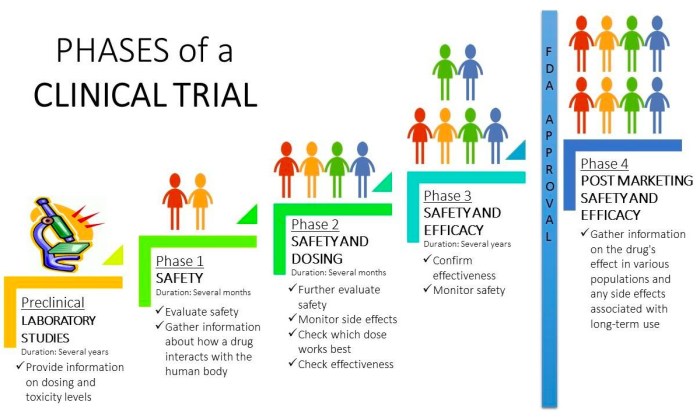
Phases of Clinical Trials
Clinical trials are typically conducted in four phases, each with specific objectives and participant characteristics. These phases build upon each other, progressively assessing the treatment’s safety and efficacy in larger and more diverse populations.
| Phase | Objective | Participants | Duration |
|---|---|---|---|
| Phase I | Assess safety, dosage, and side effects in a small group of healthy volunteers or patients. | 20-100 healthy volunteers or patients with the disease. | Several months |
| Phase II | Evaluate the effectiveness and further assess safety in a larger group of patients with the disease. | 100-300 patients with the disease. | Several months to 2 years |
| Phase III | Confirm effectiveness, monitor side effects, compare to standard treatments in a large group of patients. | 300-3000+ patients with the disease, often randomized and controlled. | 1-4 years |
| Phase IV | Monitor long-term effects, assess risks and benefits in a broader population after market approval. | Thousands of patients post-market approval. | Ongoing |
Types of Clinical Trial Designs
Various clinical trial designs exist, each suited to different research questions and objectives. The choice of design significantly influences the strength and validity of the resulting conclusions.
One of the most common designs is the randomized controlled trial (RCT). In an RCT, participants are randomly assigned to either an experimental treatment group or a control group (e.g., receiving a placebo or standard treatment). This randomization helps minimize bias and ensures that any observed differences between groups are likely due to the treatment itself. For example, a large-scale RCT might compare a new drug for hypertension against a currently used medication to determine if the new drug is more effective or has fewer side effects.
Another important design is the observational study. In contrast to RCTs, observational studies do not involve random assignment. Researchers observe and collect data on participants without intervening in their treatment. These studies are often used to investigate the long-term effects of treatments or to explore the association between risk factors and disease outcomes. For example, a cohort study might follow a group of patients who have received a particular treatment over many years to assess their long-term survival rates and the occurrence of adverse events.
The Role of Medical Devices in Clinical Trials

Medical devices, unlike pharmaceuticals, encompass a broad range of products designed for diagnosis, treatment, or prevention of disease. Their evaluation within clinical trials differs significantly from drug trials, necessitating specialized methodologies and regulatory pathways. This section explores the unique aspects of medical device clinical trials, focusing on their evaluation, regulatory landscape, and approval processes.
Medical Device Evaluation in Clinical Trials
The evaluation of medical devices in clinical trials hinges on demonstrating safety and effectiveness for their intended use. This often involves a multi-phased approach, starting with pre-clinical testing (e.g., bench testing, animal studies) to assess basic functionality and biocompatibility. Subsequent clinical trials then build upon this foundation, focusing on aspects such as device performance, efficacy, and potential adverse events in human subjects.
These trials are rigorously designed to address specific clinical questions related to the device’s intended use, and the data collected is meticulously analyzed to support regulatory submissions. The specific design of a medical device trial depends heavily on the device’s classification (Class I, II, or III, based on risk), with higher-risk devices requiring more extensive clinical evidence.
Regulatory Pathways for Medical Devices versus Pharmaceuticals
The regulatory pathways for medical devices and pharmaceuticals differ substantially. Pharmaceutical development follows a linear path, typically involving phases I, II, and III clinical trials culminating in a New Drug Application (NDA) submission. Medical device regulation, however, is more nuanced, employing a risk-based approach. The regulatory pathway is determined by the device’s classification and intended use, ranging from less stringent requirements for low-risk Class I devices to extensive clinical trials and rigorous premarket approval (PMA) for high-risk Class III devices.
While both pathways require rigorous scientific evidence, the specific requirements and the type of evidence needed differ considerably. For instance, a Class III device might need to undergo multiple pivotal clinical trials demonstrating superiority to existing treatments, similar to a Phase III drug trial, while a Class I device may only require limited testing.
Obtaining Regulatory Approvals for Medical Devices
Securing regulatory approval for a medical device is a complex process that varies depending on the device’s classification and the regulatory body involved (e.g., FDA in the US, EMA in Europe). Generally, it involves submitting a comprehensive application package, including pre-clinical data, clinical trial results, manufacturing information, and risk assessment. For high-risk devices requiring PMA, the regulatory agency will conduct a thorough review of the application, potentially requesting additional information or clarification.
This review process can be lengthy, often taking several years. Once approved, the device can be marketed and sold, but post-market surveillance is crucial to monitor its performance and identify any potential safety issues. Continuous vigilance and reporting of adverse events are mandatory throughout the device’s lifecycle.
Flowchart Illustrating the Steps Involved in a Medical Device Clinical Trial
The following flowchart depicts a simplified representation of the steps involved in a medical device clinical trial. Note that the specific steps and their order may vary based on the device’s classification and the specific clinical question being addressed.[Imagine a flowchart here. The flowchart would begin with “Idea Generation/Device Design,” followed by “Pre-clinical Testing,” then branching into “Investigational Device Exemption (IDE) Application” and “Ethics Committee Approval.” The next step would be “Clinical Trial Protocol Development,” followed by “Patient Recruitment and Enrollment,” “Data Collection and Analysis,” “Report Writing,” and finally “Regulatory Submission (510(k) or PMA).” There would be feedback loops and potential branching paths reflecting the iterative nature of the process and the possibility of needing amendments or additional studies.]
Data Management and Analysis in Clinical Trials
The success of a clinical trial hinges significantly on the meticulous management and rigorous analysis of the collected data. Robust data management systems are crucial for maintaining data integrity, ensuring accuracy, and facilitating efficient analysis, ultimately leading to reliable and trustworthy results that can inform clinical practice and guide future research. Without a well-structured system, the risk of errors, inconsistencies, and biases increases dramatically, potentially compromising the entire trial and its conclusions.
Effective data management and analysis involves a complex interplay of processes and statistical techniques. From data collection and entry to cleaning, validation, and ultimately, statistical analysis, each step requires careful planning and execution to minimize errors and ensure the reliability of the findings. This process is particularly critical given the potential impact of clinical trial results on patient care and healthcare policy.
Statistical Methods Used in Clinical Trial Data Analysis
Clinical trial data analysis employs a wide array of statistical methods, chosen based on the study design, the type of data collected, and the research question. Commonly used methods include t-tests for comparing the means of two groups, analysis of variance (ANOVA) for comparing means across multiple groups, and regression analysis for exploring relationships between variables. For time-to-event data, survival analysis techniques such as the Kaplan-Meier method and Cox proportional hazards model are frequently employed.
Furthermore, more sophisticated methods like mixed-effects models might be used to account for the correlation between repeated measurements within the same subject. The selection of the appropriate statistical method is paramount to ensure the validity and interpretability of the results. For instance, a randomized controlled trial comparing a new drug to a placebo might use a t-test to compare the mean improvement in a symptom score between the two groups.
Conversely, a study investigating the impact of multiple risk factors on disease progression might utilize a regression model.
Potential Sources of Bias in Clinical Trial Data Analysis
Bias can significantly distort the results of a clinical trial, leading to inaccurate conclusions. Several sources of bias can creep into the analysis phase. Selection bias, for example, can occur if the participants in the study are not representative of the target population. Measurement bias can arise from inconsistencies in how data is collected or recorded. Recall bias, often seen in retrospective studies, can result from participants’ inaccurate recollections of past events.
Finally, reporting bias can occur if certain results are selectively reported or emphasized while others are downplayed or omitted. Minimizing bias requires careful study design, rigorous data collection protocols, and the use of appropriate statistical methods to account for potential confounding factors. For example, blinding participants and assessors to treatment allocation can help reduce bias related to subjective assessments.
Best Practices for Ensuring Data Integrity in Clinical Trials
Maintaining data integrity is paramount in clinical trials. Several best practices can help ensure this:
The following points are critical to uphold data integrity throughout the clinical trial process:
- Establish a comprehensive data management plan: This plan should detail all aspects of data handling, from collection and entry to cleaning, validation, and storage. It should include procedures for handling missing data and dealing with inconsistencies.
- Use validated data entry systems: These systems help prevent errors and ensure data consistency. Data entry should ideally be double-checked to minimize errors.
- Implement rigorous data validation checks: These checks should identify and correct errors and inconsistencies in the data before analysis. This might include range checks, consistency checks, and plausibility checks.
- Maintain detailed audit trails: These trails document all changes made to the data, ensuring transparency and accountability.
- Use appropriate statistical methods: The choice of statistical methods should be justified and appropriate for the study design and type of data. Sensitivity analyses should be conducted to assess the robustness of the results to different assumptions.
- Employ independent data monitoring committees: These committees provide an independent oversight of the data and help ensure the integrity of the trial.
The Interplay of Medical Devices, Medical Research, and Medical Services
Medical devices are inextricably linked to both medical research and the delivery of medical services. Advancements in one area directly influence the others, creating a continuous cycle of innovation and improvement in healthcare. This interplay is crucial for developing effective treatments, improving patient outcomes, and advancing the overall quality of medical care.The integration of medical devices into medical research is multifaceted.
Devices serve as essential tools in research studies, enabling data collection, monitoring physiological parameters, and delivering therapies. Simultaneously, the findings from medical research drive the design, development, and refinement of new medical devices. This dynamic relationship ensures that medical devices are continually evolving to meet the ever-changing needs of patients and healthcare providers.
Roles of Healthcare Professionals in Device Development and Implementation
Physicians play a critical role in identifying unmet clinical needs, guiding research directions, and evaluating the efficacy and safety of new devices. Researchers, often scientists and engineers, are responsible for the design, development, and testing of the devices. Technicians are essential in maintaining, operating, and troubleshooting the devices in both research and clinical settings. Effective collaboration between these professionals is crucial for successful device development and implementation.
The contributions of each professional group are distinct yet complementary, ensuring the creation and deployment of safe and effective medical technology.
Examples of Medical Device Integration
The following table illustrates the interconnectedness of medical devices, medical research, and medical services through several examples.
| Medical Device | Related Medical Research | Impact on Medical Services |
|---|---|---|
| Implantable Cardioverter Defibrillator (ICD) | Research on cardiac arrhythmias, electrophysiology, and miniaturization of electronic components. | Improved survival rates for patients with life-threatening arrhythmias; more effective management of heart failure. |
| Insulin Pump | Research on diabetes management, glucose monitoring technology, and automated insulin delivery systems. | Improved glycemic control for patients with diabetes; reduced risk of complications; increased patient autonomy. |
| Minimally Invasive Surgical Instruments (Laparoscopic instruments) | Research on surgical techniques, biomaterials, and robotics. | Reduced surgical trauma; shorter hospital stays; faster patient recovery; improved cosmetic outcomes. |
| Artificial Joint Replacements (Hip, Knee) | Research on biocompatibility, materials science, and joint biomechanics. | Improved mobility and quality of life for patients with osteoarthritis; reduced pain and disability. |
| Cochlear Implants | Research on auditory neuroscience, signal processing, and biocompatibility. | Improved hearing and communication for patients with profound hearing loss; enhanced quality of life. |
In conclusion, clinical trials represent a critical bridge between scientific discovery and improved patient care. Their rigorous methodology, ethical considerations, and multifaceted approach ensure the development of safe and effective medical interventions. The collaborative efforts of researchers, healthcare professionals, and participants are essential to advancing medical knowledge and improving health outcomes worldwide. The ongoing evolution of clinical trial design and data analysis techniques promises even more impactful advancements in the future.
Question & Answer Hub
What is the difference between a randomized controlled trial (RCT) and an observational study?
RCTs randomly assign participants to different treatment groups, while observational studies simply observe participants without intervention, making RCTs better for establishing cause-and-effect relationships.
How long does a clinical trial typically take?
The duration varies greatly depending on the phase and complexity of the trial, ranging from a few months to several years.
Who can participate in a clinical trial?
Eligibility criteria vary depending on the specific trial but often include factors like age, health status, and the disease being studied.
Are clinical trials safe?
Extensive safety measures are in place to protect participants. Ethical review boards oversee the trials, and rigorous monitoring ensures participant safety is prioritized.
How can I find out about clinical trials?
ClinicalTrials.gov is a publicly accessible database that lists ongoing and completed clinical trials worldwide. You can also ask your doctor about relevant trials.

