Navigating the complex landscape of medical device regulations is crucial for manufacturers aiming for global market access. These regulations, designed to ensure patient safety and product efficacy, vary significantly across jurisdictions, demanding a thorough understanding of applicable standards and processes. Failure to comply can result in significant financial penalties, market withdrawals, and reputational damage, highlighting the critical need for proactive regulatory strategy.
This overview explores the key regulatory bodies, premarket and postmarket requirements, and the evolving challenges presented by emerging technologies. We will delve into the intricacies of various approval pathways, highlighting best practices for maintaining compliance throughout the entire product lifecycle, from initial design to post-market surveillance. The impact of these regulations on medical device design, development, and the broader healthcare ecosystem will also be examined.
Introduction to Medical Device Regulatory Requirements
The global medical device industry operates under a complex web of regulations designed to ensure patient safety and product efficacy. These regulations, while sometimes perceived as burdensome, are fundamentally crucial for maintaining public trust and preventing potentially catastrophic consequences. Understanding these requirements is paramount for manufacturers, distributors, and healthcare providers alike.Medical device regulations are not static; they evolve in response to technological advancements, emerging safety concerns, and evolving societal expectations.
A thorough understanding of their historical context and current landscape is essential for navigating the complexities of this vital industry.
A Brief History of Major Regulatory Milestones
The evolution of medical device regulation has been a gradual process, driven by significant incidents and a growing awareness of the potential risks associated with poorly designed or manufactured devices. Early regulations often focused on specific device types or addressed immediate safety concerns. However, the late 20th century saw the emergence of more comprehensive and harmonized regulatory frameworks.
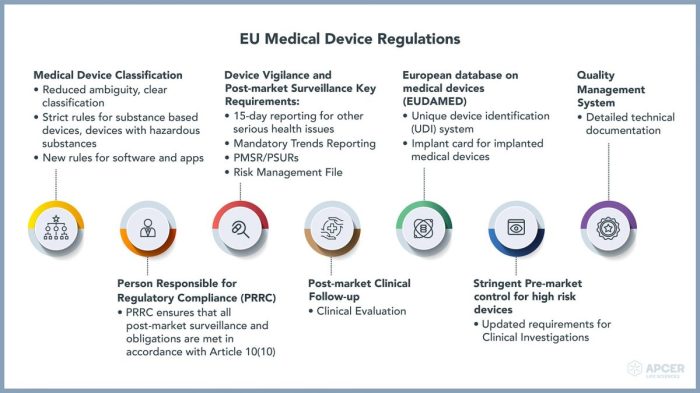
Key milestones include the establishment of the United States Food and Drug Administration (FDA) in 1906, the Medical Device Amendments of 1976 in the US, which significantly strengthened regulations, and the creation of the European Union’s Medical Device Directive (MDD) in 1993, later replaced by the Medical Device Regulation (MDR) in 2017. These legislative acts established a foundation for global regulatory cooperation, although significant variations remain between countries and regions.
Examples of Non-Compliance Impacts
Non-compliance with medical device regulations can lead to a range of severe consequences, impacting both the companies involved and, critically, patients. Financial penalties, ranging from substantial fines to complete product recalls, are common outcomes. More seriously, non-compliance can result in product liability lawsuits, potentially leading to significant financial losses and reputational damage. In extreme cases, faulty or unsafe devices can cause serious injury or even death to patients, leading to criminal investigations and devastating legal ramifications.
For example, the recall of faulty hip implants in the early 2010s resulted in millions of dollars in costs for the manufacturer, extensive litigation, and significant harm to patients. This case highlights the devastating human and financial consequences of regulatory non-compliance. Another example involves the consequences faced by companies whose devices were found to have inadequate sterilization processes, leading to infections and patient deaths.
These incidents demonstrate the high stakes associated with failing to meet regulatory requirements.
Postmarket Surveillance and Reporting

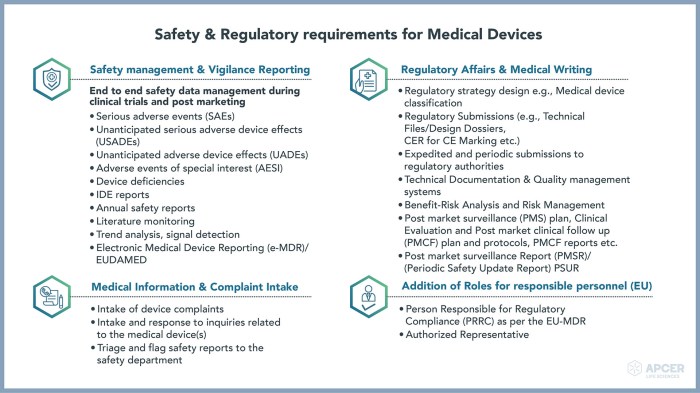
Postmarket surveillance is a critical aspect of medical device regulation, ensuring the ongoing safety and effectiveness of devices after they’ve been released to the market. It involves a continuous monitoring process designed to detect potential problems and implement corrective actions swiftly. This process is mandated by regulatory bodies worldwide and is essential for maintaining public health and protecting patients.Postmarket surveillance activities encompass a range of procedures, from collecting data on device performance to responding to adverse events and initiating product recalls when necessary.
These activities are crucial for identifying emerging safety concerns, assessing the long-term effects of device use, and improving device design and manufacturing processes. Failure to adequately conduct postmarket surveillance can lead to serious consequences, including patient harm and regulatory sanctions.
Postmarket Surveillance Activities Requirements
Regulatory bodies like the FDA (in the US) and the EMA (in Europe) Artikel specific requirements for postmarket surveillance. These requirements vary depending on the device’s classification (Class I, II, or III, for example, reflecting increasing risk), but generally involve establishing a postmarket surveillance plan, collecting and analyzing data on device performance, and submitting periodic reports to the regulatory authorities.
The plan should detail the methods used for data collection, the frequency of reporting, and the criteria for triggering investigations or corrective actions. Data sources might include device registries, post-market studies, complaint databases, and adverse event reports. Failure to adhere to these requirements can result in regulatory enforcement actions.
Adverse Event Reporting Procedures
Adverse events (AEs), defined as any undesirable experience associated with the use of a medical device, must be reported to the appropriate regulatory authorities. The reporting process often involves a structured format, including details about the patient, the device, the event itself, and any related actions taken. Serious AEs, those that result in death, life-threatening situations, hospitalization, or permanent disability, typically require immediate reporting.
Manufacturers are usually responsible for collecting and reporting AEs, and often have internal processes in place to manage this process efficiently and effectively. Delayed or incomplete reporting can lead to significant regulatory penalties. For example, a delayed report on a faulty heart valve leading to patient death could result in substantial fines and reputational damage for the manufacturer.
Product Recall Procedures
Product recalls are initiated when a medical device poses a significant risk to patient safety. The recall process involves identifying the affected devices, notifying healthcare providers and patients, and implementing procedures to remove the devices from the market. The specific procedures vary depending on the severity of the risk and the type of device, but generally involve close collaboration with regulatory authorities.
Manufacturers are responsible for managing the recall effectively, including tracking the return of recalled devices and providing replacement or alternative solutions to affected patients. A poorly managed recall can severely damage a company’s reputation and expose it to significant legal and financial liabilities. For instance, a delayed recall of a contaminated drug delivery system could result in widespread illness and considerable legal repercussions.
Best Practices for Maintaining Regulatory Compliance Post-Market
Maintaining regulatory compliance post-market requires a proactive and comprehensive approach.
A robust post-market surveillance system is essential. This includes establishing clear procedures for collecting and analyzing data, proactively identifying potential issues, and promptly responding to any reported problems. Regular internal audits and management reviews help ensure that the system remains effective and compliant with regulatory requirements. This ensures continuous monitoring and adaptation to new findings or changes in regulatory guidelines.
Effective communication with regulatory authorities is crucial. Open and transparent communication can help build trust and ensure that any potential problems are addressed quickly and efficiently. Proactive reporting of potential issues, even minor ones, demonstrates a commitment to patient safety and regulatory compliance.
Keeping detailed records is vital for demonstrating compliance. Maintaining comprehensive documentation of all post-market activities, including surveillance data, adverse event reports, and recall procedures, is essential for demonstrating compliance with regulatory requirements during inspections or audits. This also allows for tracking and analysis of trends over time.
Successfully navigating the global regulatory landscape for medical devices requires a multifaceted approach encompassing meticulous planning, rigorous testing, and ongoing vigilance. Understanding the specific requirements of each target market, coupled with a robust quality management system, is paramount for ensuring both compliance and market success. The ever-evolving nature of medical technology necessitates continuous adaptation and proactive engagement with regulatory bodies to remain at the forefront of innovation while upholding the highest standards of patient safety.
Popular Questions
What is the difference between 510(k) clearance and PMA approval?
510(k) clearance demonstrates substantial equivalence to a legally marketed predicate device, requiring less extensive data. PMA approval, conversely, necessitates a more rigorous process, including clinical trials, for novel devices lacking predicate devices.
How often should a medical device company conduct post-market surveillance?
The frequency of post-market surveillance varies depending on the device’s risk classification and intended use. However, continuous monitoring and regular reporting of adverse events are essential for maintaining compliance and ensuring patient safety.
What are the penalties for non-compliance with medical device regulations?
Penalties for non-compliance can range from warning letters and injunctions to significant fines, product recalls, and even criminal prosecution, depending on the severity of the violation and jurisdiction.
What is a Medical Device Single Audit Program (MDSAP)?
MDSAP is a program that allows manufacturers to undergo a single audit to meet the requirements of multiple regulatory authorities, streamlining the regulatory process and reducing burdens.

