
The journey from a promising molecule to a life-saving medication is a complex and often lengthy process. Medical research and drug development encompass a vast array of disciplines, from basic scientific discovery to intricate clinical trials and rigorous regulatory approvals. This intricate process involves substantial investment, collaboration across multiple sectors, and a deep commitment to ethical considerations. This overview explores the key stages, challenges, and opportunities within this vital field, highlighting the impact on global healthcare.
From the initial identification of a potential drug target to the final marketing and distribution, each stage presents unique challenges and requires specialized expertise. We will delve into the intricacies of the drug development pipeline, examining the funding mechanisms, regulatory landscapes, and technological advancements that shape this critical area of biomedical innovation. Furthermore, we will explore the crucial role of clinical trials, data analysis, and the ethical considerations that underpin this process.
Medical Research Funding and Investment
Securing adequate funding is paramount to the advancement of medical research and the subsequent development of life-saving drugs and treatments. The complex interplay of various funding sources significantly influences the pace and direction of scientific progress, impacting everything from basic research to clinical trials. A diverse funding landscape is crucial for fostering innovation and ensuring the sustainability of the medical research ecosystem.
The sources of funding for medical research are multifaceted and often collaborative. Funding typically comes from a combination of public and private entities, each with its own priorities and funding mechanisms.
Sources of Funding for Medical Research
Medical research relies on a diverse range of funding sources to support its endeavors. These sources vary in their size, scope, and approach to funding, but all play a vital role in the overall progress of medical science.
- Government Grants: National Institutes of Health (NIH) in the United States, and equivalent agencies in other countries, provide substantial funding through competitive grant programs. These grants often support basic research, clinical trials, and the development of new technologies. The funding process is typically rigorous, involving peer review and evaluation of research proposals.
- Private Investment: Pharmaceutical companies and biotechnology firms invest heavily in research and development (R&D) to discover and develop new drugs and therapies. This investment is often driven by market potential and the prospect of generating profits from successful products. Private investment frequently focuses on translational research and clinical development.
- Philanthropic Contributions: Foundations, charities, and individual donors contribute significantly to medical research, often supporting specific diseases, research areas, or institutions. Philanthropic funding can provide crucial support for innovative research that might not otherwise be funded by government or industry.
Impact of Funding Cuts on Medical Research and Drug Development
Reductions in funding for medical research can have profound and far-reaching consequences. These cuts can lead to delays in the development of new treatments, hinder the progress of ongoing research projects, and ultimately limit the advancement of medical science. Specific impacts can include:
- Reduced research capacity: Funding cuts can force laboratories to reduce staff, limit the scope of research projects, and delay or cancel research initiatives altogether.
- Slowed drug development: Clinical trials are expensive and require significant funding. Funding cuts can directly impact the number of trials conducted and the speed at which new drugs are brought to market.
- Limited access to innovative therapies: Without adequate funding, breakthroughs in medical research may not translate into new treatments available to patients.
Successful Public-Private Partnerships in Medical Research
Collaboration between public and private entities is crucial for accelerating medical research and translating discoveries into practical applications. Successful partnerships leverage the strengths of both sectors, combining public funding and infrastructure with private sector expertise and resources. Examples of such partnerships include:
- The development of the COVID-19 vaccines: Operation Warp Speed, a U.S. government initiative, partnered with pharmaceutical companies to accelerate the development and distribution of COVID-19 vaccines. This public-private partnership dramatically reduced the timeline for vaccine development and deployment.
- The Cancer Moonshot initiative: Launched by the U.S. government, this initiative aims to accelerate cancer research and development through collaborations between government agencies, academic institutions, and private companies.
Comparative Analysis of Medical Research Funding Models
Funding models for medical research vary significantly across different countries. These differences reflect national priorities, healthcare systems, and economic conditions. For example, some countries prioritize government funding, while others rely more heavily on private investment. The United States, for instance, has a large, government-funded research enterprise, whereas some European countries may have a greater emphasis on public-private partnerships.
A comprehensive comparison would require detailed analysis of specific national policies and funding mechanisms, but it is clear that diverse models exist, each with its strengths and weaknesses in terms of promoting research innovation and translating it into tangible health benefits.
The Intersection of Medical Research, Drug Development, and Medical Services
The seamless integration of medical research, drug development, and medical services is crucial for translating scientific breakthroughs into tangible improvements in patient care. This intricate relationship involves a complex interplay of innovation, regulation, and economic factors, ultimately shaping the accessibility and affordability of advanced healthcare. Effective collaboration across these sectors is essential for optimizing healthcare outcomes and ensuring equitable access to life-saving treatments and technologies.
Advancements in medical research directly influence the quality and effectiveness of medical services. New diagnostic tools, refined surgical techniques, and innovative therapies all stem from rigorous research efforts. This continuous cycle of discovery and application leads to improved patient outcomes, reduced mortality rates, and enhanced overall quality of life. The translation of research findings into clinical practice, however, is not always immediate and requires careful consideration of various factors, including regulatory approval, cost-effectiveness, and widespread adoption by healthcare professionals.
Impact of New Drugs and Medical Devices on Healthcare Costs and Access
The introduction of new drugs and medical devices often has a significant impact on healthcare costs and access. While these innovations can lead to improved treatment outcomes and potentially reduced long-term costs through preventing complications or reducing hospital stays, the initial costs of developing, manufacturing, and distributing these technologies can be substantial. This can lead to high prices for patients and increased financial burdens on healthcare systems.
Furthermore, the availability of new treatments may not be equally distributed, creating disparities in access based on factors such as geographic location, socioeconomic status, and insurance coverage. For example, the high cost of novel cancer therapies has resulted in limited access for many patients, highlighting the ongoing challenge of balancing innovation with affordability and equitable access. Conversely, the development of cost-effective generic medications has significantly improved access to essential treatments, demonstrating the potential for positive impacts on both cost and access.
Examples of Successful Collaborations
Several successful collaborations exemplify the synergistic potential of integrating research, development, and clinical practice. The development of mRNA vaccines for COVID-19, for instance, showcased remarkable collaboration between academic research institutions, biotechnology companies, and regulatory agencies. Rapid progress in research, coupled with streamlined regulatory processes and efficient manufacturing capabilities, enabled the rapid deployment of these vaccines globally, significantly impacting the pandemic’s trajectory.
Similarly, collaborations between pharmaceutical companies and healthcare providers in clinical trials facilitate the efficient testing and validation of new drugs and therapies, ensuring that research findings are effectively translated into improved clinical practice. These partnerships also foster the development of clinical guidelines and best practices, which contribute to standardization and improvement in healthcare delivery.
Factors Influencing the Adoption of New Medical Technologies
The adoption of new medical technologies in healthcare settings is influenced by a multitude of interconnected factors. Cost-effectiveness is a primary concern, with healthcare systems and providers often weighing the potential benefits against the financial implications of implementing new technologies. Regulatory approvals and safety concerns play a significant role, as rigorous testing and evaluation are necessary to ensure the safety and efficacy of new treatments.
The availability of trained personnel to operate and maintain new technologies is also crucial, as is the integration of these technologies into existing healthcare workflows. Furthermore, physician acceptance and patient preferences are important factors, as successful implementation requires the active participation and endorsement of both healthcare professionals and patients. Finally, reimbursement policies and insurance coverage can significantly influence the adoption rate of new medical technologies.
The absence of adequate reimbursement mechanisms may hinder the widespread adoption of even the most promising innovations.
Challenges and Opportunities in Medical Research and Drug Development

The medical research and drug development industry, while crucial for improving human health, faces significant hurdles in its quest to bring innovative therapies to patients. These challenges, coupled with the transformative potential of emerging technologies, create a complex landscape of both obstacles and exciting possibilities. Understanding these dynamics is critical for optimizing the efficiency and impact of future research efforts.
Major Challenges in Medical Research and Drug Development
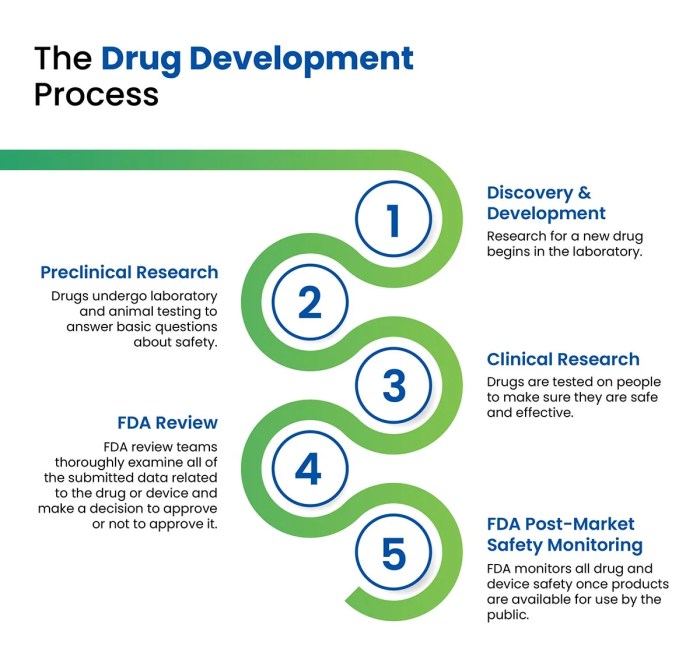
The path from initial research concept to a marketable drug is notoriously long and expensive. Several key challenges consistently impede progress.
- High Costs: The financial investment required for research, clinical trials, regulatory approval, and manufacturing is substantial, often exceeding billions of dollars for a single drug. This restricts access for smaller companies and limits the number of projects pursued.
- Lengthy Timelines: The drug development process can take over a decade, encompassing extensive preclinical testing, multiple phases of clinical trials, and rigorous regulatory review. This protracted timeline delays patient access to potentially life-saving treatments.
- Regulatory Hurdles: Navigating the complex regulatory landscape, including obtaining approvals from agencies like the FDA (in the US) and EMA (in Europe), requires significant time, resources, and expertise. Stringent regulations are essential for patient safety, but they can also slow down the development process.
- Failure Rates: A significant proportion of drug candidates fail during preclinical or clinical development, leading to substantial financial losses and setbacks for researchers and investors. This high attrition rate highlights the inherent risks associated with drug development.
- Translational Challenges: Translating promising preclinical research findings into successful clinical outcomes remains a significant obstacle. Differences between animal models and human physiology often lead to unexpected results and therapeutic failures.
Innovative Approaches to Overcome Challenges
Addressing the challenges Artikeld above requires a multifaceted approach involving technological innovation, process optimization, and collaborative efforts.
One promising strategy is the adoption of artificial intelligence (AI) and machine learning (ML) in drug discovery. AI algorithms can analyze vast datasets to identify potential drug candidates, predict their efficacy and safety, and optimize clinical trial design. This can significantly reduce the time and cost associated with traditional drug development approaches. For example, Atomwise uses AI to screen millions of molecules for potential drug interactions, dramatically accelerating the early stages of drug discovery.
Another key area is the development of innovative clinical trial designs. Adaptive trials, for instance, allow researchers to modify the trial protocol based on accumulating data, leading to more efficient and cost-effective studies. Furthermore, the increased use of biomarkers can help identify patients most likely to respond to a specific treatment, leading to smaller, more focused clinical trials.
Examples of Successful Strategies for Accelerating Drug Development
The development of mRNA vaccines for COVID-19 serves as a powerful example of accelerated drug development. Leveraging existing research on mRNA technology and employing a collaborative, globally coordinated approach, scientists were able to develop and deploy effective vaccines within an unprecedented timeframe. This success highlighted the potential of rapid technology transfer, streamlined regulatory processes, and global collaboration in overcoming traditional bottlenecks.
Similarly, the use of CRISPR-Cas9 gene editing technology holds immense promise for accelerating the development of targeted therapies for genetic diseases. This technology allows researchers to precisely modify genes involved in disease pathogenesis, offering the potential for curative treatments. While still in its early stages, CRISPR-Cas9 technology has already shown remarkable progress in treating certain types of cancer and genetic disorders.
Opportunities Presented by Emerging Technologies and Changing Healthcare Landscapes
The convergence of several factors presents significant opportunities for advancement. The rise of personalized medicine, driven by advances in genomics and proteomics, allows for the development of tailored therapies based on an individual’s genetic makeup. This approach promises improved efficacy and reduced side effects compared to traditional “one-size-fits-all” treatments.
Furthermore, the increasing availability of large-scale datasets, coupled with advances in data analytics, is revolutionizing drug discovery and development. The ability to analyze vast amounts of patient data, genomic information, and clinical trial results can identify new drug targets, predict treatment outcomes, and optimize clinical trial design. The integration of these data sources promises to significantly improve the efficiency and effectiveness of the entire drug development pipeline.
In conclusion, medical research and drug development represent a dynamic and multifaceted field, constantly evolving with technological advancements and shifting healthcare needs. While significant challenges remain, particularly concerning costs and timelines, the potential for transformative breakthroughs continues to drive innovation. The collaborative efforts of researchers, pharmaceutical companies, regulatory bodies, and healthcare providers are essential to ensuring that new treatments and technologies reach patients who need them most, ultimately improving global health outcomes.
Detailed FAQs
What is the average cost of developing a new drug?
The cost varies greatly depending on the drug type and complexity, but estimates typically range from hundreds of millions to over a billion dollars.
How long does it typically take to develop a new drug?
The timeline can span 10-15 years or more, encompassing research, development, testing, and regulatory approval.
What are orphan drugs?
Orphan drugs are medications developed to treat rare diseases affecting a small population. They often receive special regulatory considerations and incentives.
What role does intellectual property play in drug development?
Patents and other forms of intellectual property protection are crucial for incentivizing investment and protecting the innovations of pharmaceutical companies.

